Introduction: Somatic mutations (SM) are central to the pathophysiology of myelodyspastic syndromes (MDS). Iron overload (IOL) from transfusion of red blood cells (RBC) and the oxidative stress that results from the ability of iron to transfer electrons and undergo Fenton chemistry are common and are associated with adverse clinical endpoints (infections, cardiac events, exacerbation of bone marrow failure, event-free survival, progression-free survival, overall survival) in MDS. We reviewed the literature to elucidate what evidence is available indicating an interaction between iron, oxidative stress and specific SM in MDS.
Methods: A series of PubMed searches were done including the key words of each specific mutation combined with iron, oxidative stress, and reactive oxygen species (ROS, a measure of oxidative stress), focusing on SM prevalent in MDS and/or included in the molecular International Prognostic Scoring System (IPSS-M; 31 mutations in all, including IPSS-M mutations and TET2). Citations relevant to hematopoietic stem and progenitor cells, cells of the hematopoietic microenvironment, and myeloid disorders were favoured for review. Mutations of familial predisposing conditions were also searched in combination with iron, oxidative stress and ROS.
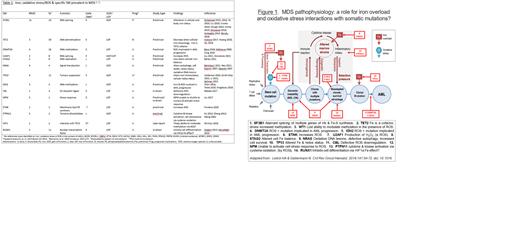
Results: Of 31 mutations found in the IPSS-M, an additional four mutations found in familial predisposing conditions (DDX41, GATA2, CHEK2, SAMD9) were searched as was TET2, for a total of 35 mutations. Fifty-four references were identified. Fifty-three references were preclinical/translational in nature, with one case report (WT1). The most frequent alterations in iron/ROS were with the SF3B1 mutation, with 11 citations. Thirteen of 31 (42%) of SM in combination with iron, oxidative stress/ROS had citations. Reports for combination with iron or oxidative stress/ROS, respectively, included early (TP53, IDH2, RUNX1, SF3B1, STAG2, TET2; and TP53, DNMT3A, IDH2, NPM1, U2AF1) and late (NRAS, CEBP3A; and CBL, NRAS) mutations. Both gain of function (TP53, IDH2, NRAS, SF3B1; and TP53, IDH2, NRAS, U2AF1) and loss of function (STAG2, TET2; and CBL) mutations had relevant citations identified. Cellular pathways affected included tumour suppressor (TP53 for both iron and oxidative stress/ROS), signal transduction (NRAS for both), splicing factors (SF3B1; U2AF1), DNA stability (STAG2) and (hypo) methylation (TET2; DNMT3A). There were reports of an impact at multiple levels on iron/ROS and cellular/systemic physiology in the context of SF3B1 mutation, for example: ERFE+12 (containing 12 additional nucleotides and translated to an ERFE protein containing 4 additional amino acids; 358 vs 354 in wild type) present in SF3B1 mutated MDS does like the usual ERFE suppress hepcidin, impacting iron at a systemic level; enzymes of iron, heme and iron sulfur cluster physiology are altered, impacting iron physiology at a cellular level; and mitochondrial ferritin sequesters iron, leading to cytoplasmic iron deficiency, lowering ROS and thus possibly protecting cells from oxidative damage. Table 1 shows examples of iron/ROS interactions with SF3B1, TET2, DNMT3A, U2AF1, STAG2, NRAS, TP53, IDH2, CBL, NPM, ETNK, PTPN11, WT1 and RUNX1. Figure 1 shows MDS pathophysiology and where interactions between SM and iron/oxidative stress/ROS may impact this pathophysiology. No investigations were identified relevant to the mutations of familial predisposing conditions, including DDX41, GATA2, CHEK2 and SAMD9 in combination with iron/oxidative stress/ROS.
Discussion: Searching IOL, oxidative stress and ROS in combination with MDS somatic mutations uncovered information as to how iron/redox status may interact with SM to affect MDS pathophysiology. Of particular interest is the cytoplasmic iron deficiency induced in the presence of SF3B1 mutation, the only SM conferring favourable prognosis in MDS in the IPSS-M. Given the pleiotropic cellular, intracellular and subcellular mechanisms involved, investigation regarding when and how targeting iron overload by reducing RBC transfusion requirements, binding iron or attenuating ROS may alter cellular and clinical outcomes in the setting of specific somatic mutations is warranted.
Disclosures
Davis:Taiho Canada: Honoraria. Leitch:Forma: Research Funding; Novartis Canada: Honoraria; Astra Zeneca Canada: Honoraria; Bristol-Myers Squibb Canada: Honoraria; Taiho Canada: Honoraria; Fibrogen: Research Funding; Janssen: Honoraria; BeiGene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal